Before we get started with an Automated QMS, let’s refresh our understanding of a generic QMS, and its benefits. To summarize:
- QMS provides a structure and framework to design a Quality Management System that will help you enhance your Productivity of the entire organization leading to Customer Delight, and ultimately Profitability through consistent Quality savings in th Quality process.
- The core elements of the framework are:
- Understanding the needs of your market/customer.
- Designing and implementing the functions of the organization as a network needed to produce quality product for your market.
- Designing and implementing feedback loops within the functions to help monitor, correct, control, and enhance each function in a network.
Rules and guidelines for doing the above are provided in various QMS Standard, ISO 9001 Quality Management System being the basis for such standards.

The Elements of QMS Quality Management System Automation
Throughout history, and especially over the last century and a half during the four Industrial Revolutions, the pace and nature of the evolution has accelerated in an exponential manner. At the end of the day, the common denominator is to minimize human effort and labor through the use of Quality Management Software.
- The first phase was to minimize use of muscle power for effort: first of animals, and then humans. Examples: transporting products in a factory, shaping metals into starting, intermediate, and final products.
- Progressively, the types of effort needed for moving product through the Organizational Network became more and more of the mental type. A notable start was type-writers to replace scribes. Then came the phase of organizing, storage, and retrieval of paper documents and records.
- This is where modern QMS came into being (1987).
- In 2024, after 36 years, we see that most companies have moved to electronic documents and records, and are using Microsoft Office applications for organizing, storage and retrieval such artifacts.
- Where we are now is on the threshold of a vast opportunity to automate the work-flows required for a QMS, releasing human time and effort to do what only humans can do: make decisions. Even in this there can be some degree of automation.
- The Automation Imperative: Since automation results in minimizing human effort, it also saves the cost of labor. However, in the Fourth (current) Industrial Revolution, it is not just the direct and obvious $$ cost saving that generates economic benefits for the company. Automation for the sake of automation is the new principle. The reason is that there are so many not-so-obvious benefits. Some of these are in improvement of Quality, Reliability, Repeatability. Automation (especially nowadays) expands an insight into discovery of hitherto unknown opportunities to improve consistent Quality by building it into the Quality process with the use of Quality Management Software.
- A big contributor is the access to information and data that helps humans make informed decisions and helps in breakthrough innovation.
- Automation of QMS therefore results in:
- Minimizing the labor contained in the current way of doing QMS actions and ISO 9001 certification.
- Ensuring the correct workflow of actions and actors and minimizing the labor in managing Quality.
- Providing data and information that can show trends that may uncover risks and opportunities by using Quality Management software.
- Provide data and information in a manner that enables automated analyses by using Quality Management Solution.
History of QIA and QISS- 30 Modules
Quality Institute of America, Inc. (QIA) was founded in 1994 by people who had a rich background in manufacturing engineering, manufacturing management, and finally Quality Management Systems. The journey of QIA started with Training, Consulting and Auditing. The founders realized that for a QMS to be fully effective and efficient, it needed to be computerized. So, in 2003 QIA introduced the Quality Institute Software Solution or QISS. Today we have 30 separate but integrated modules that address all aspects of QMS and QMS standards. QISS is in use in General Manufacturing, Automotive, Oil & Gas, Aerospace, Medical Devices, Laboratories and others.
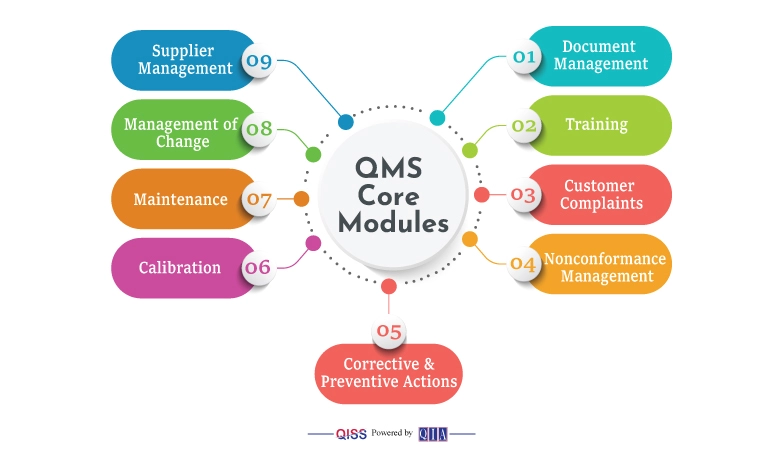
The common and consequential labor-intensive parts of a QMS are:
- Document Management
- Training.
- Customer Complaints
- Nonconformance Management
- Corrective & Preventive Actions
- Calibration
- Maintenance
- Management of Change
- Supplier Management
Below is an illustration of how QISS can help you get the maximum benefits from these. There are some labor-intensive aspects that are common to all parts of a QMS, and these are:
- Assignment of Tasks as needed by protocol.
- Managing these tasks with reminders, escalations, reassignments.
- Creating records while doing work, storing them, retrieving them.
- Gleaning meaningful data from the records to analyze them to gain actionable information, as well as intelligence on risk control and managing Quality.
- Collaborating with organizations outside of the company, such as suppliers and customers
Now, let us look at the top four parts:
Before you start using the software, though, you will need to do some system-wide setups to achieve practical QMS. This will involve assigning permissions to people to do certain tasks that they are deemed competent for. This ensures that all people have gone through appropriate training to ensure that they are in fact competent to do the jobs they need to do. There are other setups, such as those needed for Global Multi-location companies. QISS helps such companies run the QMS with a Global perspective.
Document Management:
Before you start using the Document Management module you will need to do setups relevant to this module. Such as Document Type, Standard Clauses, ect.
QISS will manage/ help manage the following aspects of Document Management:
- Creation, Review, Release and Distribution of documents:
- An author can create a document on any platform, such as Word, Excel, PDF, etc. He selects appropriate approvers from a list of authorized approvers. She will submit the draft into QISS for review and approval.
- QISS takes over the workflow from here. The selected approvers will get an e-mail alerting them that they have an approval task. They will click and read the draft.
- If some of the approvers do not approve, and wish to change it, they can do so, with any comments. QISS will return the draft to the author, who will make appropriate changes and resubmit.
- The concerned approvers get another alert to review Draft #2. They may approve, or return again in the same manner. This process will continue until all approvers approve. In the meantime, QISS keeps a full record of the back-and-forth actions in Quality Control to help in a perfect collaborative effort.
- If task assignees forget or ignore, they will be get a reminder, and you may set up an appropriate system for escalations in the Quality process.
- You may set up advance notification of due dates to help task assignees.
- When all approvers approve, author will do final approval, and set a release date, which can be immediate. He will select people to form a distribution list who will get an alert that they have a new document.
- He can restrict access to documents, such as confidential ones. Restricted access can be for individuals, departments, or groups.
- You may link other documents in the document library to the new document as an “Associated Document”
- Document numbers are assigned by QISS using an Intelligent numbering system that can help understand the nature of a document without opening it. However, you may bypass this feature and assign your own numbers manually.
- Training in a Document.
- Authorized people can assign people to be trained in a document. This training can be an instructor led “classroom” training or for people to read individually, take a test, and be certified as Trained in the document.
- Revising a Document.
- The process is similar to creating a new document. The revised document will be revved up.
- Authorized people may bypass the formal revision process for minor changes (such as grammatical errors).
- QISS will store superseded revisions for reference, with a pop-up warning alerting people to beware that they are not looking at the latest.
- Obsoleting a Document.
- Authorized people can obsolute a document, and if needed, delete it.
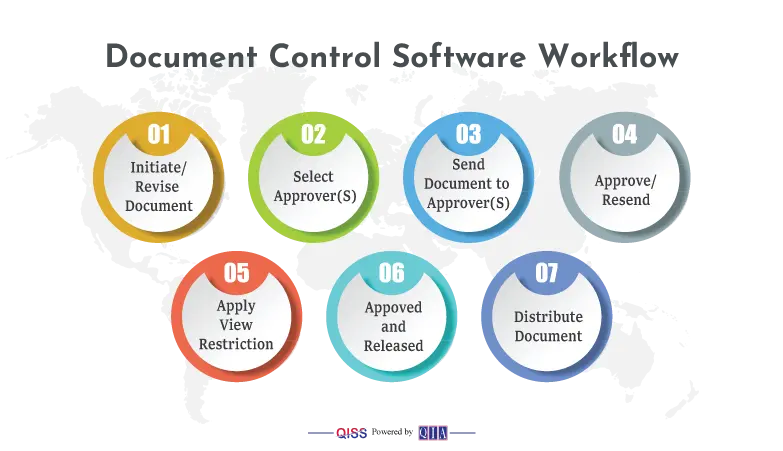
- You may share your approved documents with customers and suppliers through secure portals.
- Other Document Management features:
- QISS comes with predefined fields, however, you can use the powerful “Form Builder” to add to these fields. The new fields will have same level of functionality (such as search) as the original ones.
- You can assign different levels to your documents for easier organization—such as Level 1 for Policies, 2 for System Level Procedures, 3 for Instructions, etc.
- QISS can insert water-mark with Doc # and Rev #. Useful if you must print hard copies.
- QISS can schedule Periodic Reviews of an approved document for continuous “goodness”.
- Dashboard with charts and reports for you to assess the health of your document management system.
- Audit Trails.
- People familiar with the requirements of watertight document control system will see that the above system will make it impossible for you to fail in an audit for ISO 9001 Certification.
- Notice the QMS automation power of QISS. Humans are asked to do things that only humans can do—make decisions unique to the situation. Humans will not have to send e-mails, attaching documents, chasing people to remind them to be not tardy, Ensure that people are properly trained, and on and on. This is the power of automation that QISS brings to you in practical QMS.
Hope you agree that the above is reason enough to invest half an hour of your time for a demo with one of our engineers, live. Click here to make it happen!
Corrective and Preventive Action (CAPA):
The Corrective and Preventive Action (CAPA) module in your ISO 9001 Quality Management System is your organization’s secret weapon for continuous improvement. It helps you address issues effectively and proactively prevent them from happening again.
For an automated Quality Management Software like QISS to ensure smooth operation, you’ll need to set up some basics like user roles, task priority and input names. You can also customize the module with specific fields relevant to your organization.
The CAPA module in QISS smoothly guides you through a structured workflow in the quality process to address and prevent issues. Let’s break it down in a straightforward manner:
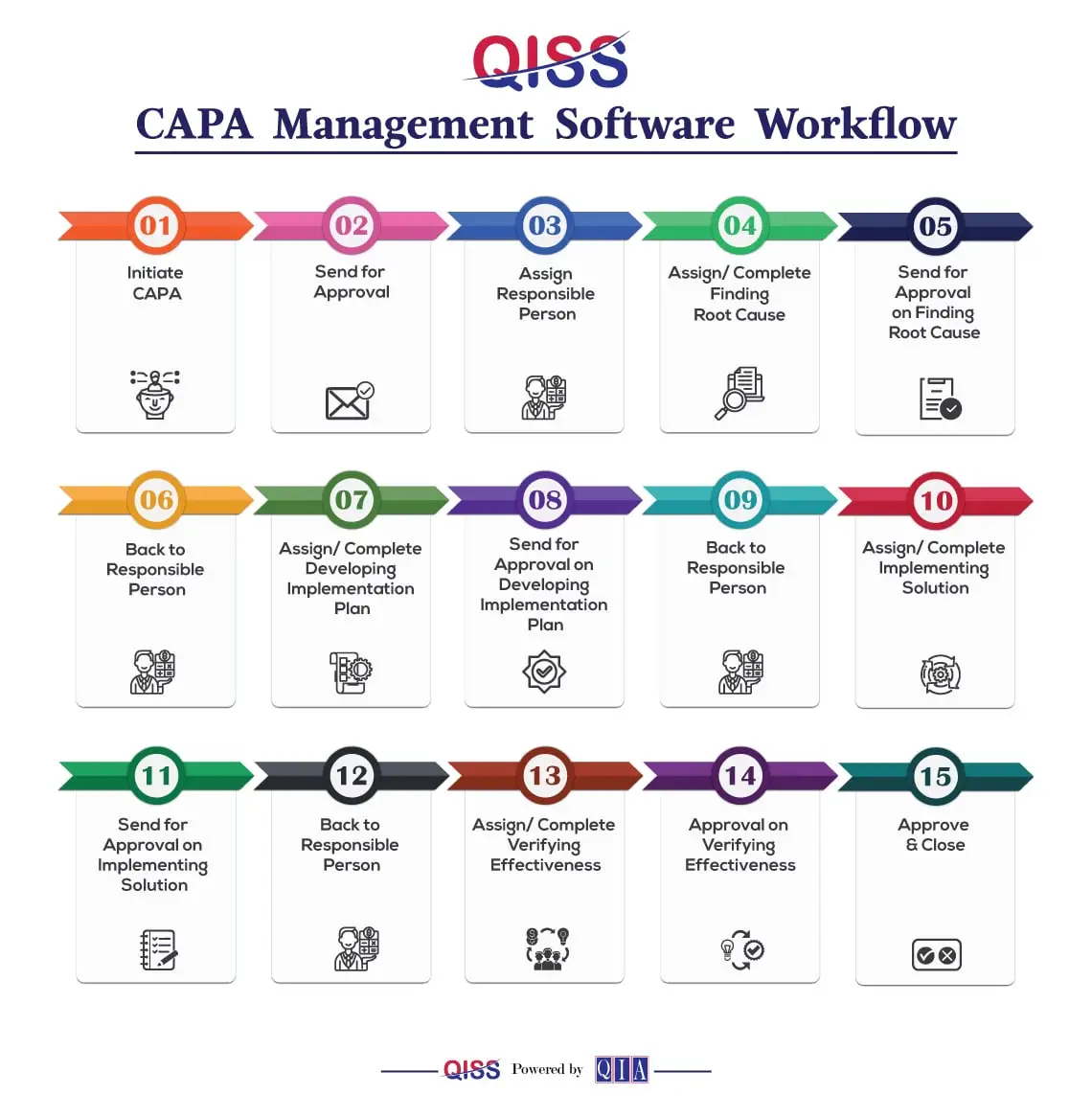
- Initiate CAPA: Begin the CAPA journey by identifying an issue that needs attention, either from an existing Non-Conformance Report or by recognizing potential risks.
- Send for Approval: Once the issue is identified, the proposed CAPA is sent for approval to ensure alignment with the broader objectives and strategies.
- Assign Responsible Person: Specify a responsible person who will take charge of the CAPA process, ensuring accountability from start to finish.
- Assign/Complete Finding Root Cause: Dive into understanding the root cause of the issue. Collaborate with the responsible person to identify and document the core problem.
- Send for Approval on Finding Root Cause: Seek approval on the identified root cause before moving forward in the CAPA process.
- Back to Responsible Person: Responsibilities circle back to the assigned person, ready to implement the next steps based on the approved root cause.
- Assign/Complete Developing Implementation Plan: Develop a comprehensive plan to address and resolve the identified issue. Assign tasks and ensure everything is prepared for approval.
- Send for Approval on Developing Implementation Plan: Obtain approval on the implementation plan, ensuring that it aligns with organizational strategies and goals.
- Back to Responsible Person: The spotlight returns to the responsible person, ready to execute the approved implementation plan.
- Assign/Complete Implementation Solution: Implement the approved solution, ensuring all aspects are in place. Assign tasks, monitor progress, and make sure everything is on track.
- Send for Approval on Implementation Solution: Seek approval on the implemented solution, marking a crucial phase in the CAPA journey.
- Back to Responsible Person: Responsibilities once again rest with the assigned person for the final stages of the CAPA process.
- Assign/Complete Verifying Effectiveness: Verify the effectiveness of the implemented solution, ensuring that the issue is resolved and won’t recur.
- Approval on Verifying Effectiveness: Obtain final approval, signaling the successful resolution of the CAPA.
- Approve & Close: Close the CAPA, acknowledging the achievement of continuous improvement through a disciplined problem-solving approach for consistent Quality Management solutions.
Features that Empower You:
- Initiate from anywhere: Start CAPAs from various sources, like NCR, identified risks, and even audits.
- Flexibility for all: Address internal issues, involve suppliers by assigning them tasks, and allow customers to initiate CAPAs, promoting collaborative improvement.
- Customization made easy: Tailor the CAPA process by defining which steps require approval, ensuring efficient progress and control.
- Clear accountability: Assign responsible individuals for each CAPA step, fostering ownership and timely completion.
- Effectiveness Follow-Up: Users can set a follow-up period after implementing a CAPA solution to re-verify effectiveness, ensuring lasting improvement.
- Stay informed: Receive automated email notifications to keep everyone updated on deadlines and progress. QISS works as your best Quality Management software in 2024 and beyond.
Additional Functionalities:
- Detailed documentation: Capture essential information throughout the process, including root cause analysis, actions taken, and preventive measures.
- Collaborative problem-solving: Facilitate communication through discussion notes and file attachments, fostering teamwork and knowledge-sharing.
- Powerful search: Easily find specific CAPAs using advanced search filters.
- Undo mistakes: Mitigate errors by reverting to previous versions, ensuring data accuracy.
- Comprehensive audit trails: Maintain a complete record of every action for transparency and regulatory compliance.
- Data-driven insights: Gain valuable insights from reports and dashboards to track progress, identify trends, and measure the effectiveness of your CAPA system.
In a nutshell, the CAPA module in QISS streamlines your journey towards continual improvement. It’s not just a tool; it’s a guide, ensuring that every step in your problem-solving process is structured, collaborative, and effective. So, embrace the power of CAPA with QISS—where challenges become opportunities, and your quality management reaches new heights.



